Abbreviations: CR: Complete response; PR: Partial response; SD: Stable... | Download Scientific Diagram

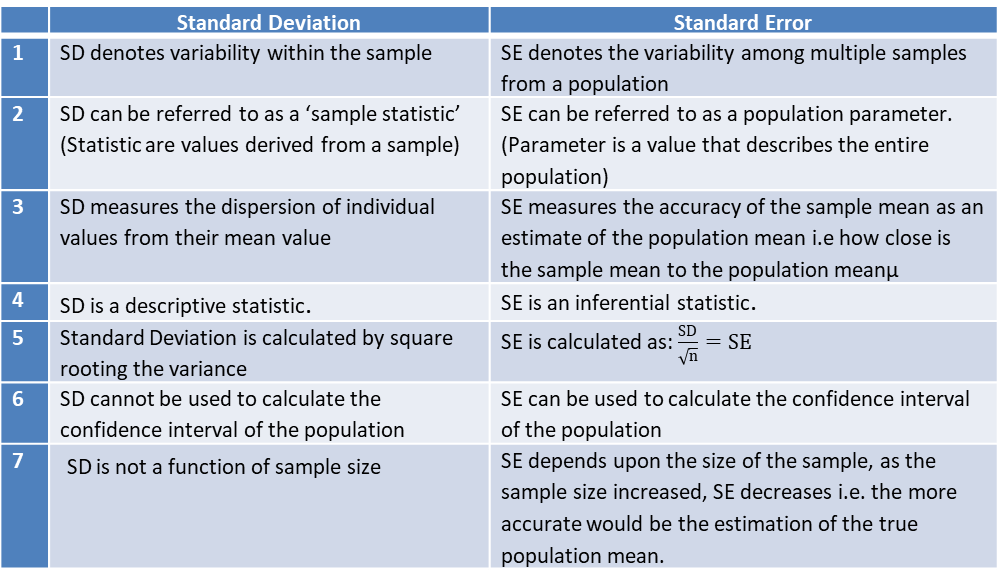
Standard Deviation and Standard Error – What is the Difference? - CliNFo.EU - ideas, tools, knowledge & best practices in clinical research

A Phase II, Open-Label Clinical Trial of Intranasal Ketamine for Depression in Patients with Cancer Receiving Palliative Care (INKeD-PC Study) - Inergency
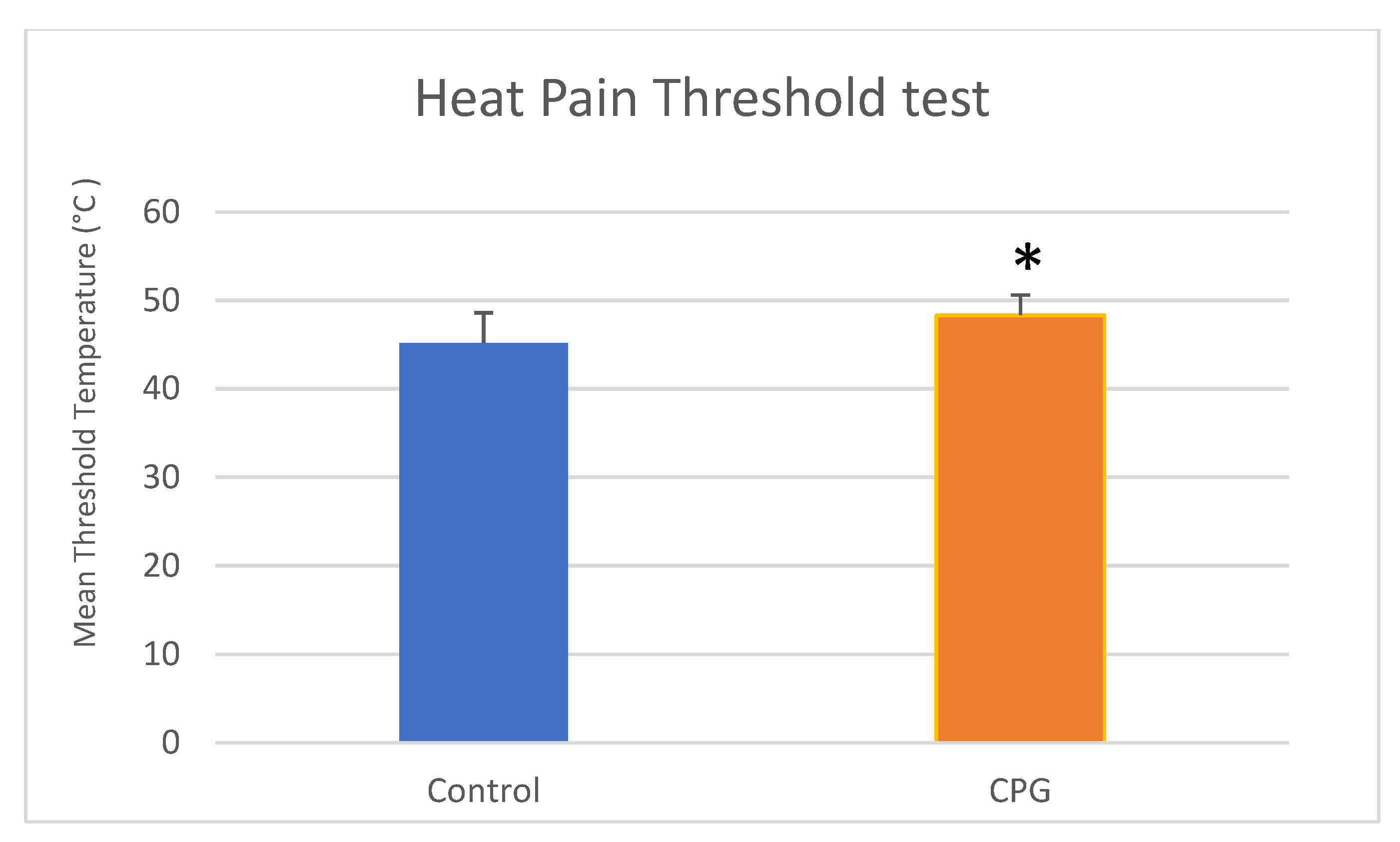
Biomedicines | Free Full-Text | Outcomes of a QST Protocol in Healthy Subjects and Chronic Pain Patients: A Controlled Clinical Trial

Clinical effectiveness and cost-effectiveness of tailored intensive liaison between primary and secondary care to identify individuals at risk of a first psychotic illness (the LEGs study): a cluster-randomised controlled trial - The

Addressing multilevel barriers to clinical trial participation among Black and White men with prostate cancer through the PACCT study - Eggly - 2023 - Cancer Medicine - Wiley Online Library

Machine learning-based quantitative prediction of drug exposure in drug-drug interactions using drug label information | npj Digital Medicine
Study Age, Mean (SD) Range BMI, Mean (SD) Current Former Never Crisafulli 2004 51.7 (4.0) 23.7 (2.7) Newton 2006 52.2 (2.4) 28.6

Clinical trial schema. Patients on ET for at least 3 months with PD or... | Download Scientific Diagram
Efficacy, tolerability, and safety of an innovative medical device for improving oral accessibility during oral examination in special-needs patients: A multicentric clinical trial | PLOS ONE
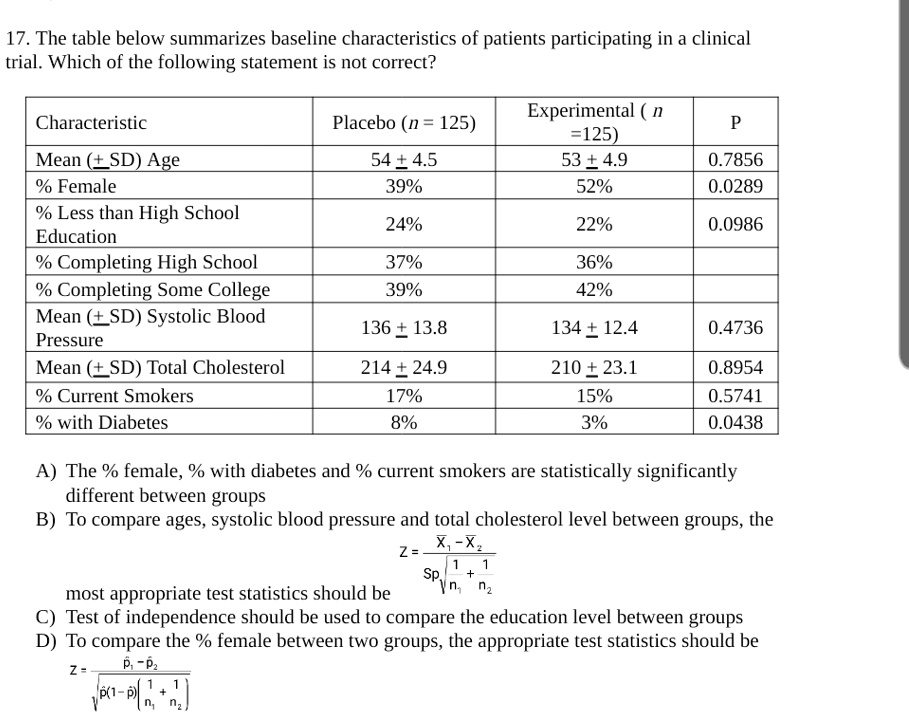
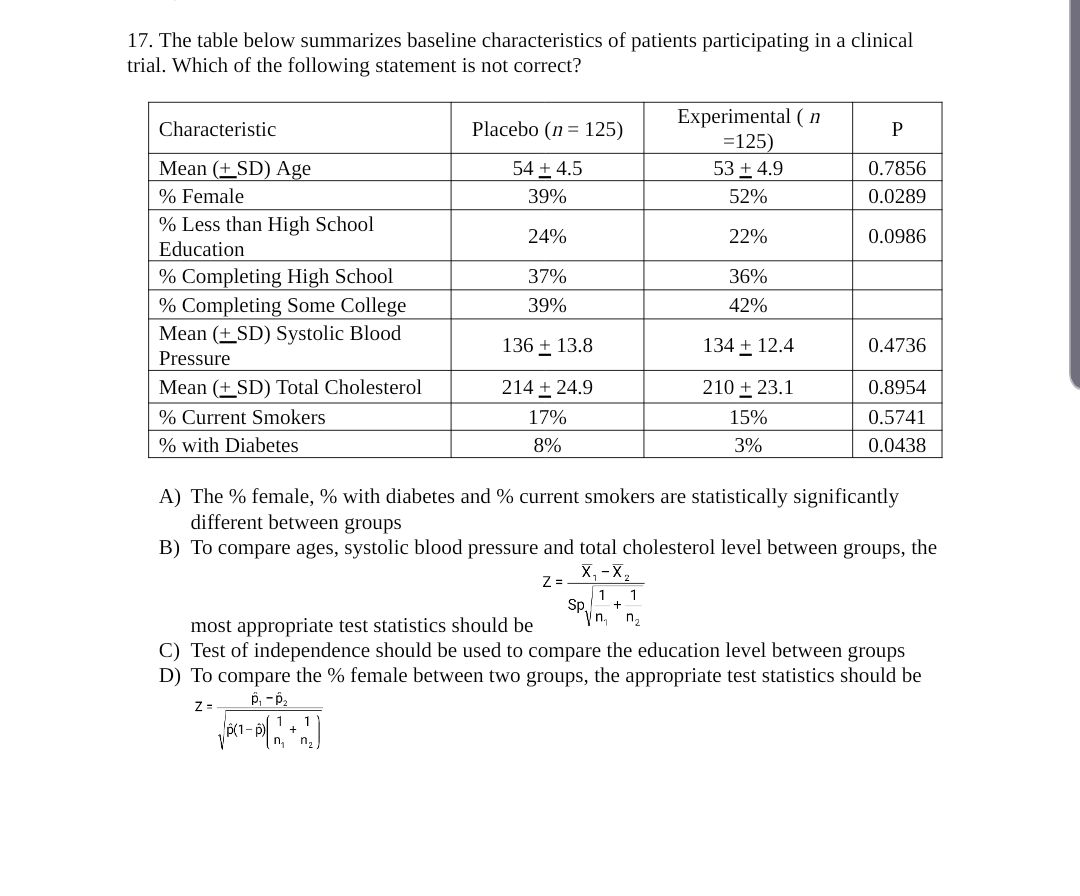
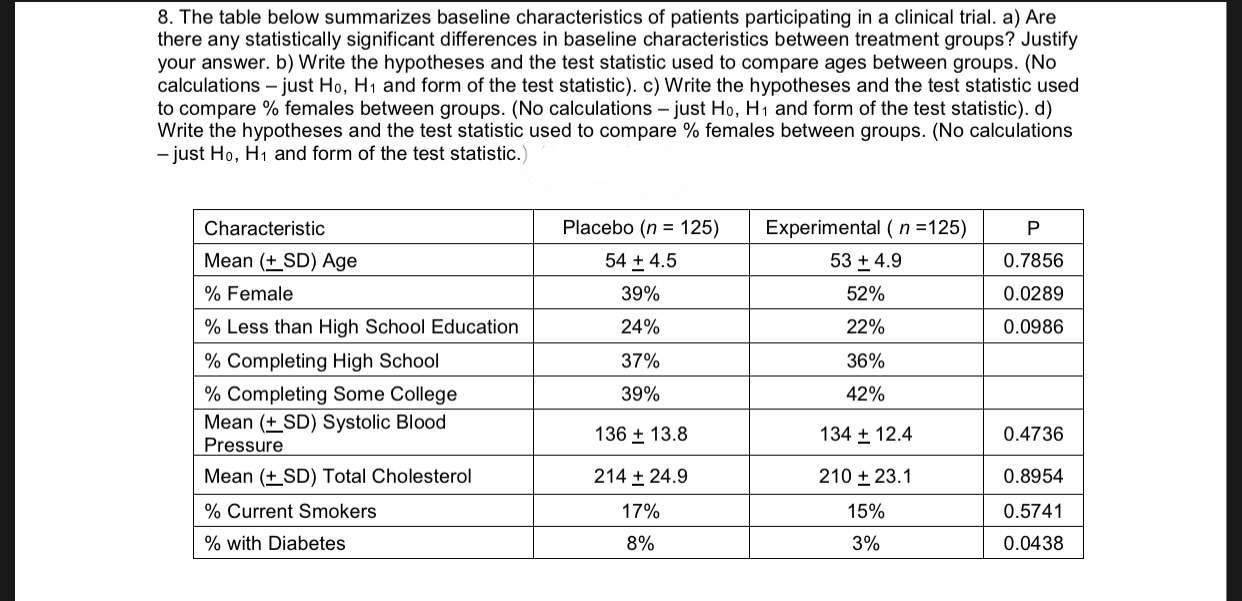
SOLVED: 17. The table below summarizes baseline characteristics of patients participating in a clinical trial. Which of the following statement is not correct? Experimental =125) 53+4.9 52% Characteristic Placebo (n = 125)
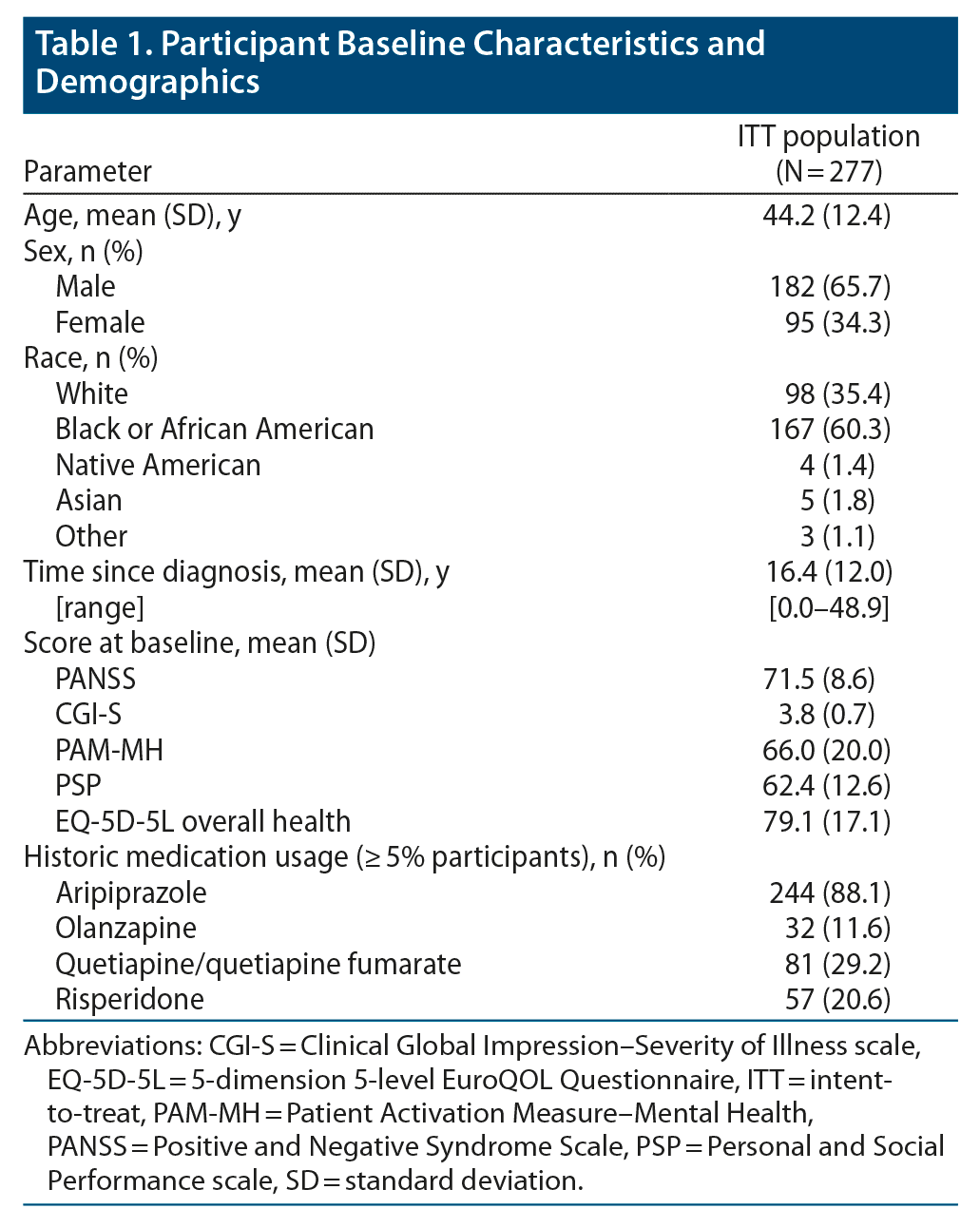
Phase 3b Multicenter, Prospective, Open-label Trial to Evaluate the Effects of a Digital Medicine System on Inpatient Psychiatric Hospitalization Rates for Adults With Schizophrenia | Psychiatrist.com
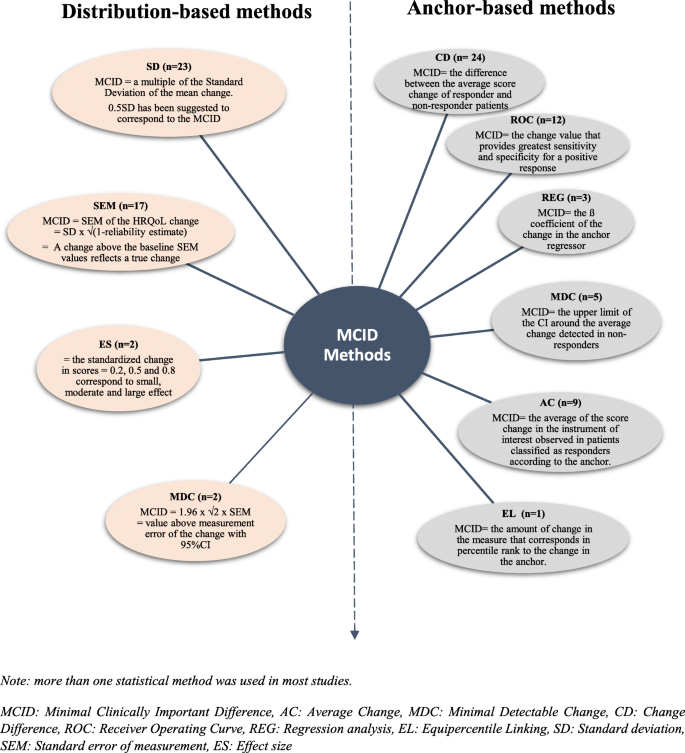
How is the minimal clinically important difference established in health-related quality of life instruments? Review of anchors and methods | Health and Quality of Life Outcomes | Full Text

Abbreviations: CR: Complete response; PR: Partial response; SD: Stable... | Download Scientific Diagram

:max_bytes(150000):strip_icc()/recist-5206400_final-04c81c00d8cc4583975603ca53a6ff98.jpg)
![PDF] The Response Shift Phenomenon in Clinical Trials | Semantic Scholar PDF] The Response Shift Phenomenon in Clinical Trials | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/c159ec244fb834650d8c8ebe25785491684513e6/2-Figure1-1.png)